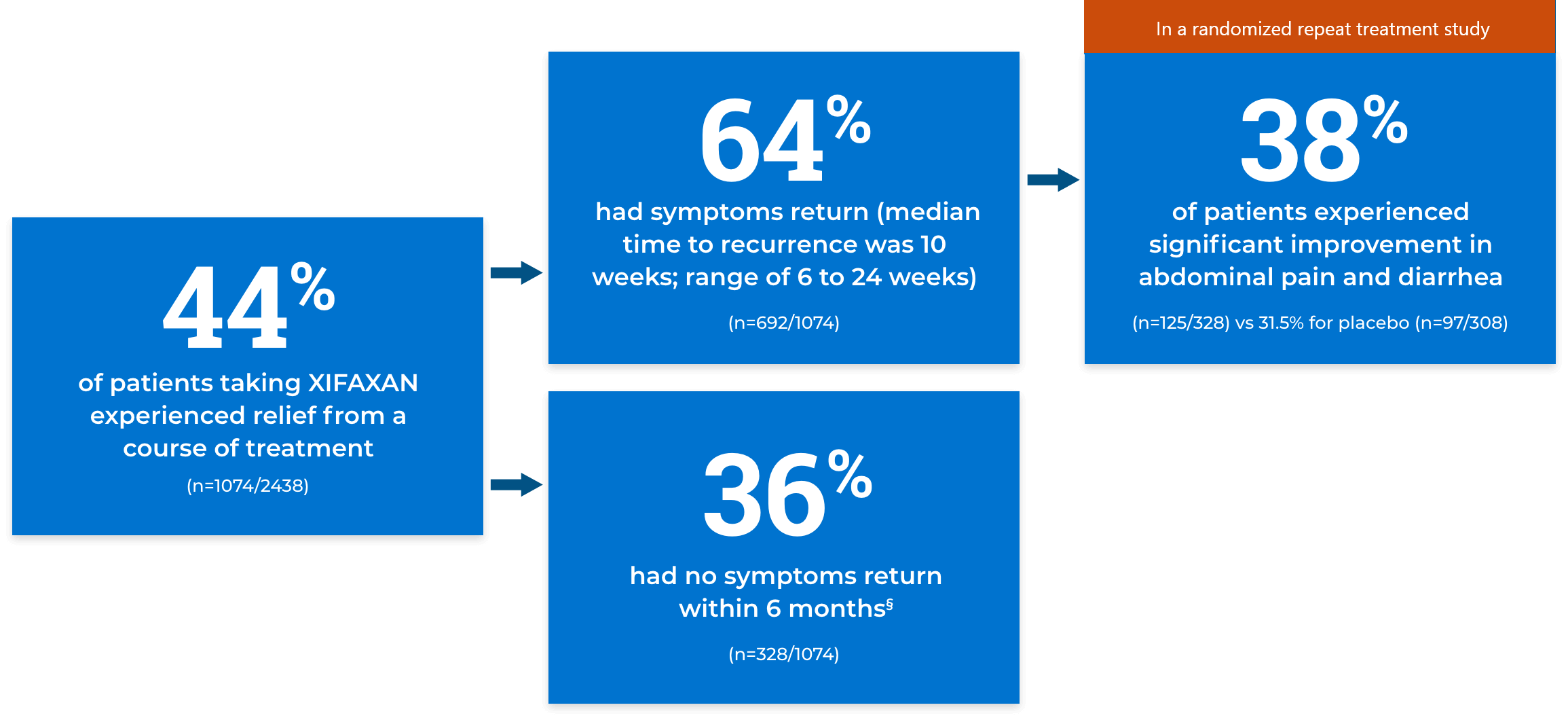
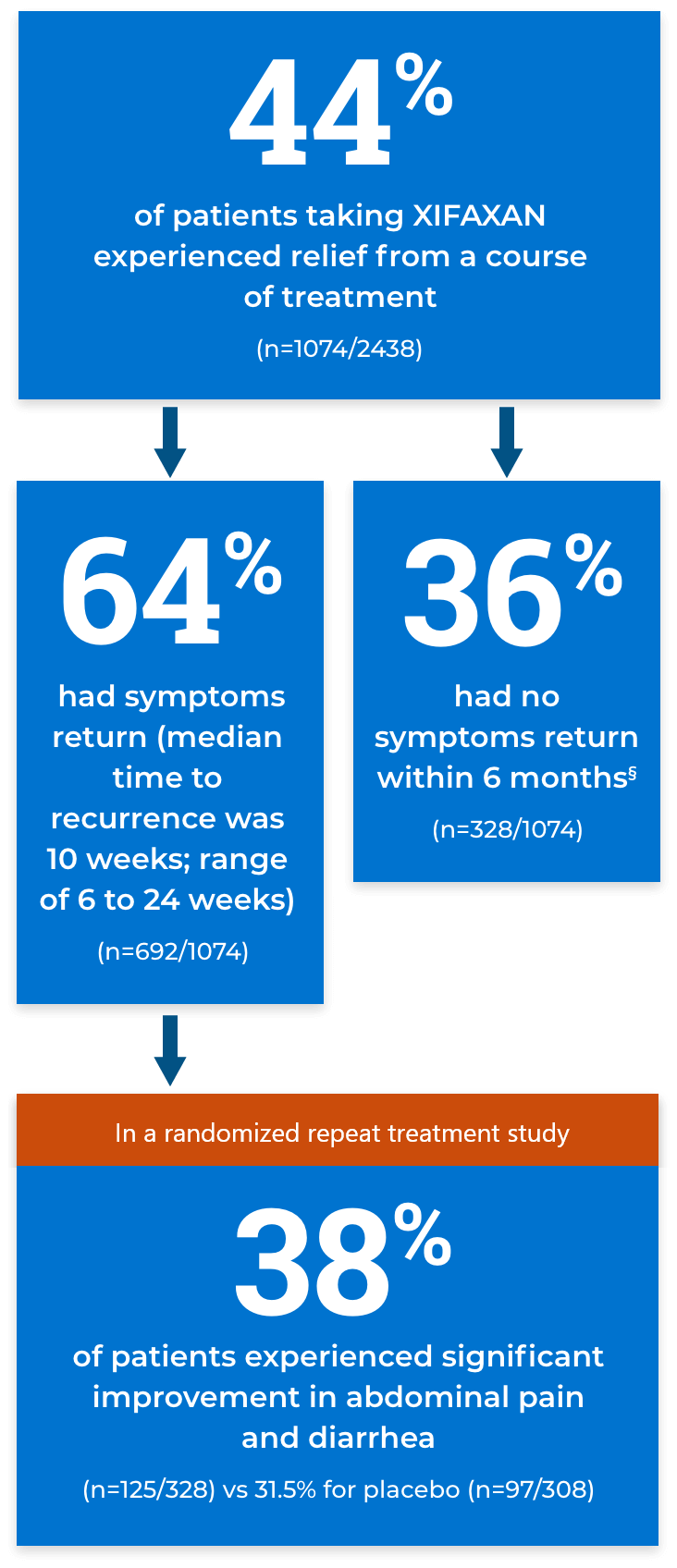
Relief from bloating
Percentage of bloating responders based on weekly responses in a pooled analysis of TARGET 1 and 2 trials.
XIFAXAN
(n=251/624)
Placebo
(n=192/634)
Key secondary endpoint: The proportion of subjects who achieved adequate relief of IBS-D–related bloating (ie, responders) for at least 2 of 4 weeks during the month following 14 days of treatment. A bloating responder was defined as a patient who responded “yes” to the weekly questions: “In regards to your IBS-D symptom of bloating, compared to the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS-D symptom of bloating? [Yes/No].”‡
‡Responses were given during the first 4 weeks of the treatment-free period following 2 weeks of active treatment (primary evaluation period).