WATCH
Dr. Jesudian discuss the real-world evidence for XIFAXAN use
SEE MORE FROM YOUR PEERS



The data presented below were gathered in a retrospective, real-world cohort study. Real-world evidence (RWE) provides data that can supplement the randomized clinical trial (RCT) findings, which remain the gold standard for evaluating the efficacy and safety of a treatment. Interpretation of the results are at the discretion of the audience as RWE data have inherent limitations.
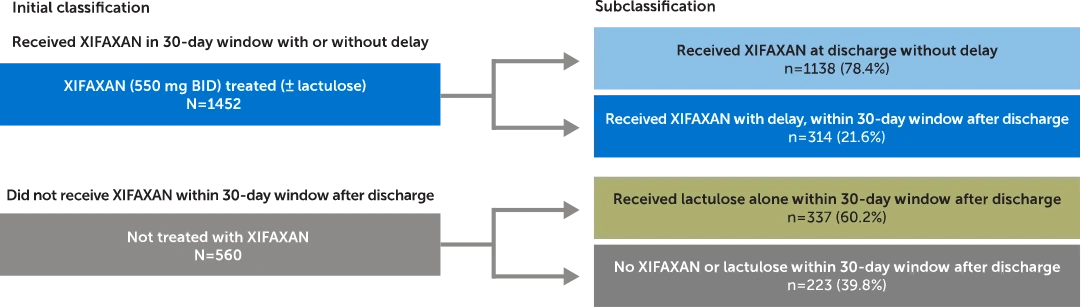
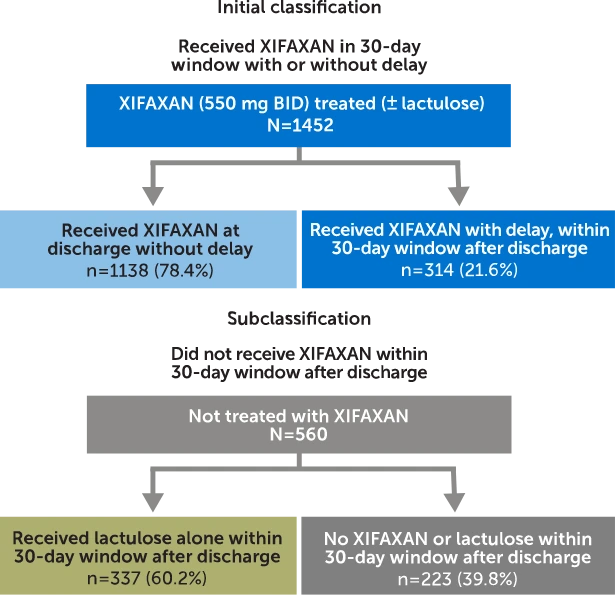
Commercially insured adults with an initial OHE hospitalization were identified and classified based on treatment received within a 30-day window after discharge and assessed for OHE rehospitalization and costs.
Claims analysis from MarketScan® Commercial Subset (October 1, 2015, through March 31, 2020). Patients were identified and classified based on treatment received in the 30-day post-discharge period. In patients who received XIFAXAN (± lactulose) within 30 days (N=1452), 78.4% (n=1138) received treatment at discharge (no delay). In patients who did not receive XIFAXAN within 30 days (N=560), 60.2% (n=337) of patients received lactulose alone.
Inclusion criteria: Adults (18-64 years old) with an initial overt OHE hospitalization* and continuous health plan enrollment‡
Exclusion criteria: Patients who had a liver transplant on or before the discharge date of the first OHE hospitalization were excluded from this study.
*Due to the lack of an OHE-specific ICD-10 code at the time of the study period to identify an OHE hospitalization in claims data, medical expertise was used to operationalize the definition of OHE hospitalization.1
†Also excluding patients who had a liver transplant on or before the discharge date of the first observed OHE hospitalization.
‡Continuous health plan enrollment ≥6 months prior to and ≥30 days after the first observed hospitalization.
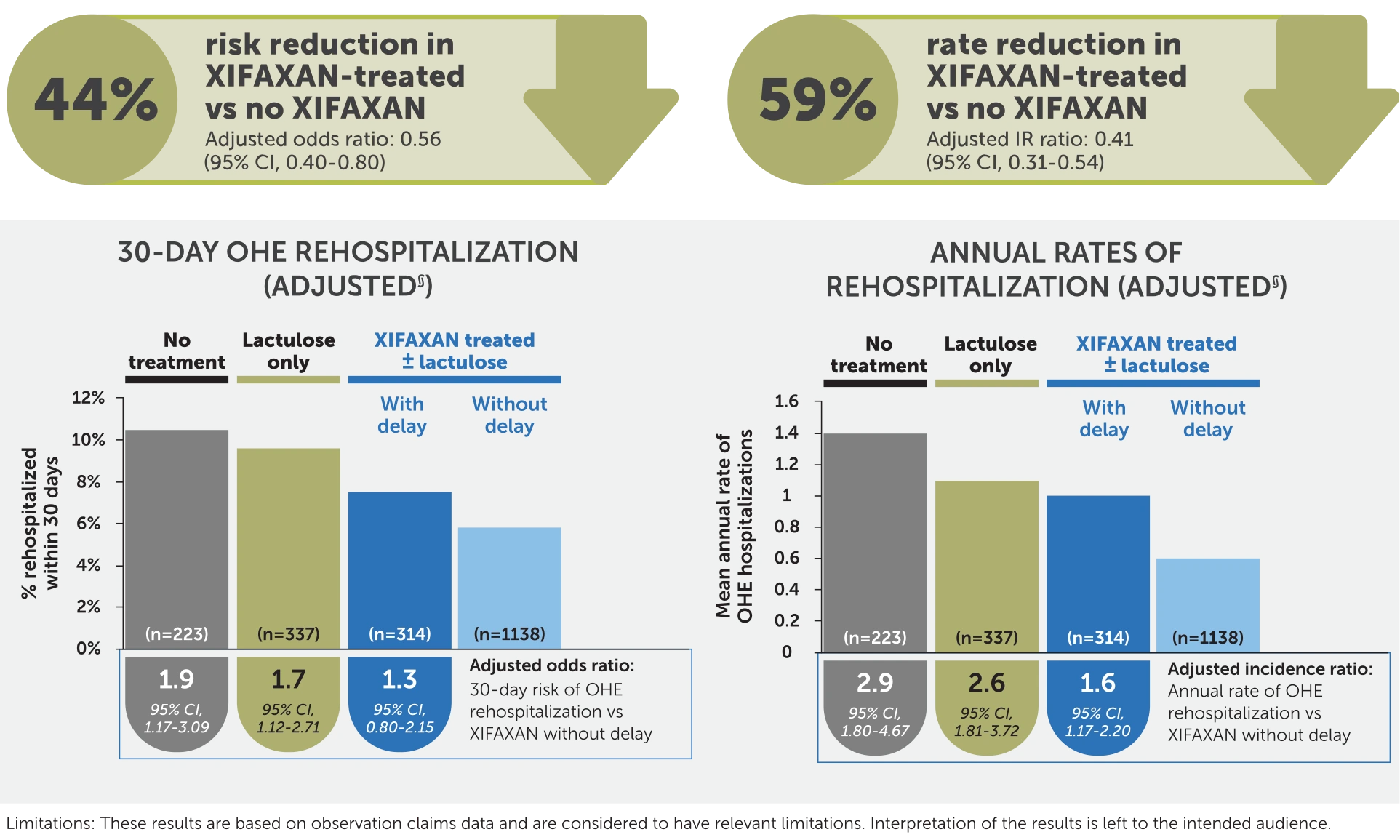
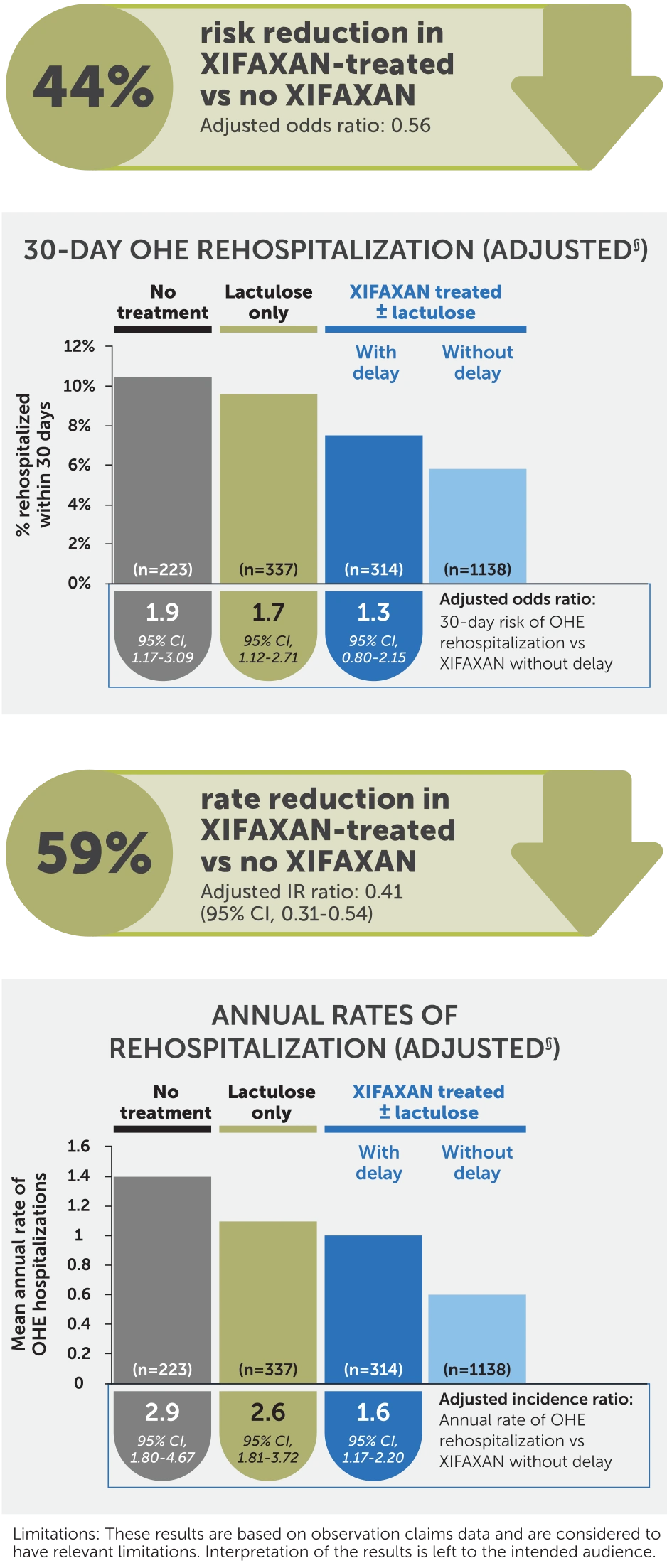
§Analyses were adjusted for a priori selected potential confounding factors associated with OHE hospitalization (ie, age, gender, health plan, region, cirrhosis-specific comorbidity scoring system score, prior gastroenterology consult, duration of index OHE hospitalization, treatment during baseline [eg, rifaximin, HE prophylaxis with antibiotics], indicators of portal hypertension [eg, varices], and factors associated with HE [eg, frailty index]).
Results are based on observational claims data and are considered to have relevant limitations:
IR, incidence rate; MELD, Model for End-Stage Liver Disease; OHE=overt hepatic encephalopathy.
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults and for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults.
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults.
References: 1. Gyawali B, Parsad S, Feinberg BA, Nabhan C. Real-world evidence and randomized studies in the precision oncology era: the right balance. JCO Precis Oncol. 2017;1:1-5. doi:10.1200/PO.17.00132 2. Jesudian AB, Gagnon-Sanschagrin P, Heimanson Z, et al. Impact of rifaximin use following an initial overt hepatic encephalopathy hospitalization on rehospitalization and costs. J Med Econ. 2023;26(1):1169-1177. doi:10.1080/13696998.2023.2255074
XIF.0256.USA.24V2.0