WATCH
Dr. Jesudian discuss some of the AASLD Guidelines for managing OHE
SEE MORE FROM YOUR PEERS



*Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE breakthrough by 58% during the 6-month treatment period.1
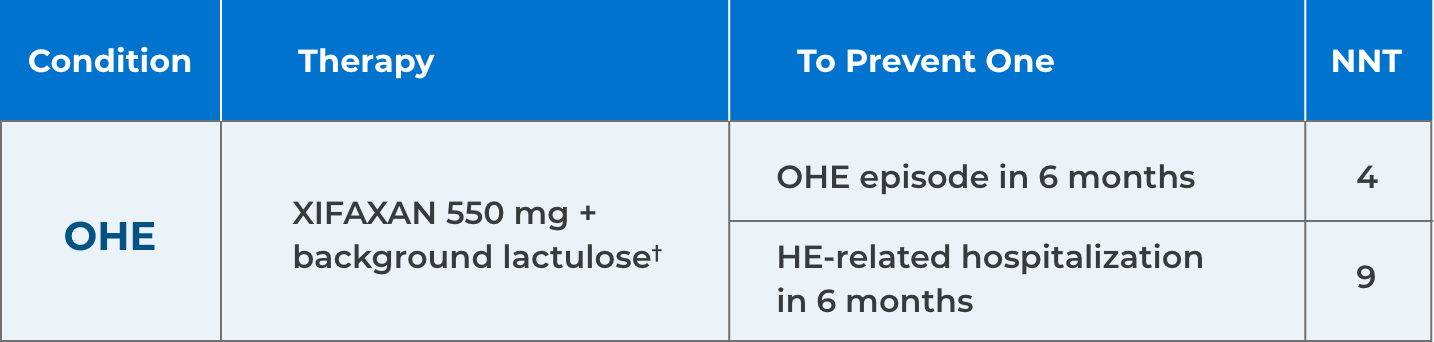
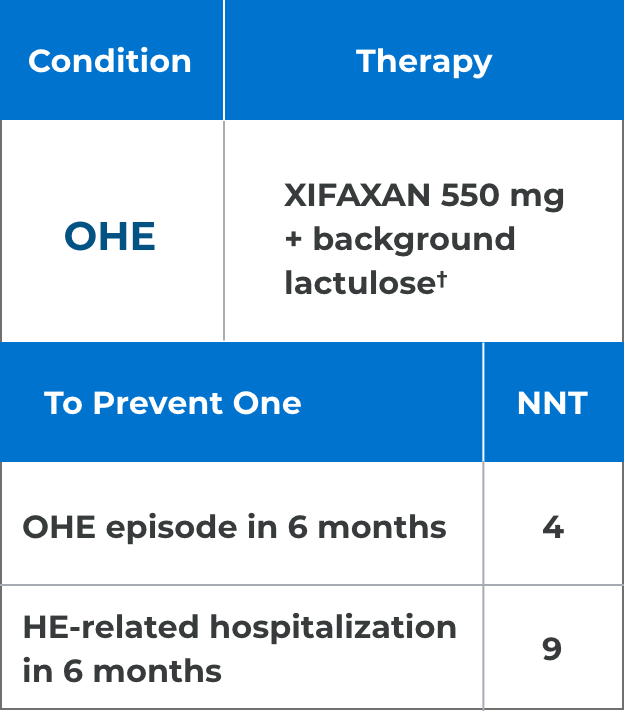
†91% of patients in the XIFAXAN group were on concomitant lactulose.1
SEE XIFAXAN
You should consider XIFAXAN as an add-on to lactulose for this patient.

You should consider XIFAXAN for this patient.
Hypothetical patients.

‡Per the GRADE System for Evidence: Grade I=randomized, controlled trials; A=evidence is “high quality,” and further research is very unlikely to change our confidence in the estimated effect; and 1=recommendation is “strong,” with factors influencing strength of recommendation including the quality of evidence, presumed patient-important outcomes, and costs.3
AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; HE, hepatic encephalopathy; OHE, overt hepatic encephalopathy; PCP, primary care physician.
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults and for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults.
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults.
References: 1. XIFAXAN. Prescribing information. Salix Pharmaceuticals; 2023. Accessed November 20, 2024. https://shared.salix.com/globalassets/pi/xifaxan550-pi.pdf 2. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071-1081. doi:10.1056/NEJMoa0907893 3. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715-735. doi:10.1002/hep.27210
XIF.0181.USA.23V5.0